
Soil removal is a complex process that is much more involved than just adding soap or surfactant to water. One of the major concerns we have in dealing with cleaning compounds is water hardness. Water is made “hard” by the presence of calcium, magnesium, iron and manganese metal ions. These metal ions interfere with the cleaning ability of detergents. The metal ions act like dirt and “use up” the surfactants, making them unavailable to act on the surface we want to clean.
A chelating agent (pronounced kee-lat-ing from the Greek word “claw”) combines itself with these disruptive metal ions in the water. The metal ions are surrounded by the claw-like chelating agent which alters the electronic charge of the metal ions from positive to negative (see diagram below.)
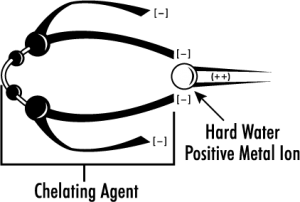
This makes it impossible for the metal ions to be precipitated with the surfactants. Thus, chelated metal ions remain tied up in solution in a harmless state where they will not use up all of the surfactants. The surfactants are then able to do their job of actually removing soil and cleaning the surface.
Our next article in this series will introduce builders and their role in cleaning.



