Home > The Chemistry of Cleaning
The Chemistry of Cleaning
Overview
What makes a cleaner work? How much chemistry is involved in removing grease from a stove top or grit from a concrete floor? The answer to these and other questions lies within words like surfactant, solvent, chelating agent and builder. Understanding the basic elements of a cleaner’s effectiveness against different types of soil is essential to the “Chemistry of Cleaning.”
Webster defines soil as “to make dirty or unclean on the surface; to foul; to dirty; to defile; as, to soil a garment with dust.” For our purposes, we will view soil as foreign matter that happens to be in the wrong place. For example, yogurt is food, but if it’s ground into carpeting, it is considered soil.
Soil Types
Soil can be broken down into three broad categories: organic, inorganic and combination.
Organic soils encompass a broad range and include food soils (such as fat, grease, protein, and carbohydrate), living matter (such as mold, yeast and bacteria) and petroleum soils (such as motor oil, axle grease and cutting oils). Traditionally, these soils have been removed using alkaline cleaners or solvents. Now, however, more modern, bio-based surfactants are used to cut organic soils.
Inorganic soils include rust, scale, hard water deposits and minerals such as sand, silt and clay. Oftentimes acids are used to remove inorganic deposits such as rust and scale. Minerals are often cleaned with general purpose or acid-type cleaner (ie. bowl cleaners).
Combination soils often present the toughest challenge for a cleaner since the soil contains both organic and inorganic components. Proper identification is critical. In the past, most combination soils were removed with a very concentrated, highly built cleaner that also contained solvent. But now, with new surfactant chemistries, it is possible to accomplish this cleaning with fewer ingredients.
Surfactants
A surfactant is the most important part of any cleaning agent. The word surfactant is short for “Surface Active Agent.” In general, they are chemicals that, when dissolved in water or another solvent, orient themselves at the interface (boundary) between the liquid and a solid (the dirt we are removing), and modify the properties of the interface.
How does a surfactant work? All have a common molecular similarity. One end of the molecule has a long nonpolar chain that is attracted to oil, grease, and dirt (the hydrophobe). Another part of the molecule is attracted to water (the hydrophile). The surfactant lines up at the interface as diagrammed below. The hydrophobic end of the molecule gets away from the water and the hydrophilic end stays next to the water. When dirt or grease is present (hydrophobic in nature) the surfactants surround it until it is dislodged from the boundary. Notice in diagram 4 that the dirt molecules are actually suspended in solution.
It should be noted that a surfactant can be either a soap or a synthetic detergent. Soaps have been used for centuries because they are made from natural materials such as animal fat and lye. Synthetics have only become widely available over the last 60 years. Soaps are still commonly used in personal hygiene products because of their mildness. Synthetic detergents are the surfactants of choice for almost all other cleaning agents.
Chelating Agents
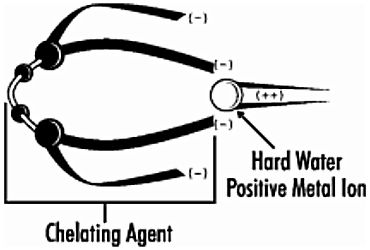
Soil removal is a complex process that is much more involved than just adding soap or surfactant to water. One of the major concerns we have in dealing with cleaning compounds is water hardness. Water is made “hard” by the presence of calcium, magnesium, iron and manganese metal ions. These metal ions interfere with the cleaning ability of detergents. The metal ions act like dirt and “use up” the surfactants, making them unavailable to act on the surface we want to clean.
A chelating agent (pronounced kee-lat-ing from the Greek word “claw”) combines itself with these disruptive metal ions in the water. The metal ions are surrounded by the claw-like chelating agent which alters the electronic charge of the metal ions from positive to negative (see diagram below.)
This makes it impossible for the metal ions to be precipitated with the surfactants. Thus, chelated metal ions remain tied up in solution in a harmless state where they will not use up all of the surfactants. The surfactants are then able to do their job of actually removing soil and cleaning the surface.
Builders
Detergents, as we have learned so far, consist of surfactants and chelating agents. Remember, surfactants remove dirt from a soiled surface and chelating agents are used to surround unwanted metal ions found in cleaning solutions. The chelating process, though very effective, is not always necessary and adds to the cost of formulating detergents. Builders are often a good alternative.
Builders are added to a cleaning compound to upgrade and protect the cleaning efficiency of the surfactant(s). Builders have a number of functions including softening, buffering, and emulsifying.
Builders soften water by deactivating hardness minerals (metal ions like calcium and magnesium). They do this one of two ways:
Sequestration – holding metal ions in solution.
Precipitation – removing metal ions from solution as insoluble materials.
Builders, in addition to softening, provide a desirable level of alkalinity (increased pH), which aids in cleaning. They also act as buffers to maintain proper alkalinity in wash water.
Finally, builders help emulsify oily and greasy soil by breaking it up into tiny globules. Many builders will actually peptize or suspend loosened dirt and keep it from settling back on the cleaned surface. Below are three of the most common builders used in today’s heavy-duty detergents. A short description of each follows.
Phosphates*usually sodium tripolyphosphate (STPP), have been used as builders extensively in heavy-duty industrial detergents. They combine with hardness minerals to form a soluble complex which is removed with the wash water. They also sequester dissolved iron and manganese which can interfere with detergency.
* Due to the potential environmental effects of phosphates in detergents, all of Essential’s newer cleaners and detergents do not contain phosphates.
Sodium carbonate (soda ash) is used as a builder but can only soften water through precipitation. Precipitated calcium and magnesium particles can build up on surfaces, especially clothing, and therefore sodium carbonate is not used in laundry detergents.
Sodium silicate serves as a builder in some detergents when used in high concentrations. When used in lower concentrations, it inhibits corrosion and adds crispness to detergent granules.
Solvents
Detergents, as we have learned so far, consist of surfactants, chelating agents and builders. Remember that surfactants are designed to remove dirt from a soiled surface. Chelating agents and builders are added to the formula to keep water hardness from interfering with the cleaning process.
Water makes up a large percentage of most liquid cleaner formulas. It is not uncommon for water-based detergents to contain 50% water or more. Some ready-to-use formulations may contain as much as 90% to 95% water! With this much water present in a cleaner, why do they work so well? Water can be considered an active ingredient that actually adds to the detergency of cleaners. It performs several very important functions in liquid cleaners. Most importantly, it adds to the “detergency” of a cleaner. Water acts as a solvent that breaks up soil particles after the surfactants reduce the surface tension and allow the water to penetrate soil. Water is capable of dissolving a variety of different substances. In fact, it is called the "universal solvent" because it dissolves more substances than any other liquid.
One can visualize how this works if you think of your own clothes washing machine. Think about what would happen if you were to add a cup of detergent to your washer and wash a load of clothes with no other water added. Your clothes certainly would not come out clean! Water is necessary for the laundry detergent to work properly.
Water also aids in the suspension and anti-redeposition of soils. Once the soil has been dissolved and emulsified away from the surface, we want to prevent it from being redeposited. Water keeps the soil suspended away from the clean surface so that it can be carried away easily during the rinsing process. It is clear that without water, our cleaning formulas would be much less effective.
In addition to water, other chemical solvents are often added to cleaners to boost performance. Compounds such as 2-Butoxyethanol (butyl), isopropyl alcohol (rubbing alcohol) and d-Limonene are all considered solvents. Their main function is to liquefy grease and oils or dissolve solid soil into very small particles so surfactants can more readily perform their function.
Preservatives
A preservative is nothing more than a substance that protects soaps and detergents against the natural effects of aging such as decay, discoloration, oxidation and bacterial degradation. Synthetic detergents are preserved differently from soaps as we will see.
In soaps, preservatives are used to forestall the natural tendency to develop rancidity and oxidize upon aging. In detergents, preservatives are used to prevent bacteria from spoiling the solution. This is necessary because detergents are naturally biodegradable by bacteria found in the air, waste treatment systems and soil once they enter the environment. Acrylics (such as those in floor finishes) can also spoil, if left untreated. Essential uses a variety of preservatives, depending on the application.